![HOTGEN antigen rapid test reagent [nasal swab test] (approved by the Hong Kong government) HOTGEN antigen rapid test reagent [nasal swab test] (approved by the Hong Kong government)](https://www.healthbuynow.com/image/cache/catalog/products/_20220413194743-550x550.jpg)
![HOTGEN antigen rapid test reagent [nasal swab test] (approved by the Hong Kong government) HOTGEN antigen rapid test reagent [nasal swab test] (approved by the Hong Kong government)](https://www.healthbuynow.com/image/cache/catalog/products/_20220413194752-550x550.jpg)
![HOTGEN antigen rapid test reagent [nasal swab test] (approved by the Hong Kong government) HOTGEN antigen rapid test reagent [nasal swab test] (approved by the Hong Kong government)](https://www.healthbuynow.com/image/cache/catalog/products/_20220413194743-80x80.jpg)
![HOTGEN antigen rapid test reagent [nasal swab test] (approved by the Hong Kong government) HOTGEN antigen rapid test reagent [nasal swab test] (approved by the Hong Kong government)](https://www.healthbuynow.com/image/cache/catalog/products/_20220413194752-80x80.jpg)
| 新冠狀病毒抗原快速測試包規格 | |
| Certification | CE Certificate EC Design-Examination Certificate Directives: 98/79/EC on In Vitro Diagnostic Medical Devices (IVDD), Annex III (6) (Devices for self-testing) No. V9 089675 0006 Rev. 00 |
| Features | High Accuracy, Specificity and Sensitivity No need instrument, get results in 15 minutes Room temperature storage |
| quantity | 1 set per box |
| sample | Human Anterior Nares Swab Detect the presence of viral proteins Identify acute or early infection |
| Sensitivity | Sensitivity: 95.37% / Specificity:99.13% / Accuracy: 97.31%. |
| 快速測試棒包含 | |
| 1. | Nasopharyngeal swab |
| 2. | sample buffer 2 |
| 3. | test card |
| 4. | Hazardous Material Storage Bag |
| 5. | Instructions for use |
| 100+ | 0% | HK$8 |
| 1000+ | -12.5% | HK$7 |
HOTGEN antigen rapid test reagent (approved by the Hong Kong government)
HOTGEN Antigen Rapid Test
(approved by the Hong Kong government)
Novel Coronavirus 2019-nCoV Antigen Test (Colloidal Gold)
Brand: Beijing Hotgen Biotech Co., Ltd.
Product Features
Stock code: 688068.SH
High Accuracy, Specificity and Sensitivity
No need instrument, get results in 15 minutes
Room temperature storage
Sample : Human Anterior Nares Swab
Detect the presence of viral proteins
Identify acute or early infection
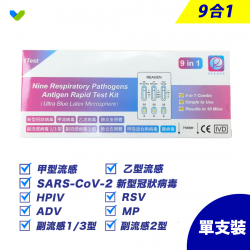
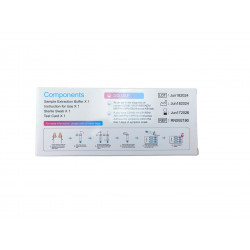
Kit includes:
Nasopharyngeal Swab / Sample Buffer / Sample Buffer / Test Card / Hazardous Material Storage Bag / Instructions for Use
Sensitivity:
95.37% / Specificity: 99.13% / Accuracy: 97.31%.
Clinical Performance
(Disease Course 5-7 Days)
CE Certificate
EC Design-Examination Certificate
Directives: 98/79/EC on In Vitro Diagnostic Medical Devices (IVDD), Annex III (6)
(Devices for self-testing)
No. V9 089675 0006 Rev. 00






























![DVOT Novel Coronavirus Detection Reagent [Salivary Test] DVOT Novel Coronavirus Detection Reagent [Salivary Test]](https://www.healthbuynow.com/image/cache/catalog/covid-check/dvot3-250x250.png)
![DVOT Novel Coronavirus Detection Reagent [Salivary Test] DVOT Novel Coronavirus Detection Reagent [Salivary Test]](https://www.healthbuynow.com/image/cache/catalog/covid-check/dvot1-250x250.png)

![Deepblue new coronavirus antigen rapid test stick [nasal swab test] Deepblue new coronavirus antigen rapid test stick [nasal swab test]](https://www.healthbuynow.com/image/cache/catalog/covid-check/1-b-250x250.png)